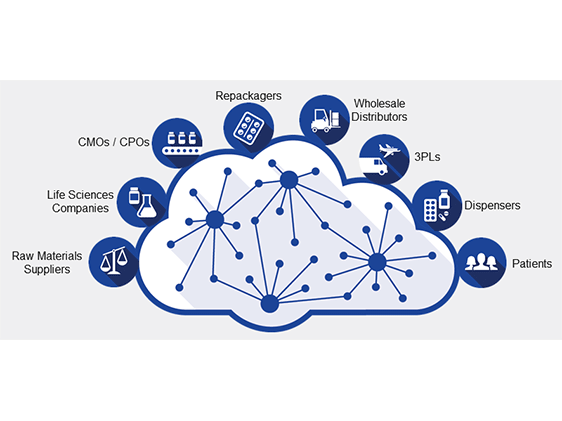
Based in North Reading MA, TraceLink is a vertical-market SaaS vendor that has developed a suite of software solutions to improve supply chain management and to support product track and trace compliance within the life science industry. The Company is currently commercializing five key product offerings, all of which operate within the TraceLink Life Sciences Cloud – a web-based platform through which the Company’s network of customers and their trading partners can seamlessly connect and interchange serialized product data.
The Company allows drug manufacturers, repackagers, distributors, wholesalers, dispensers and other constituents in the pharmaceutical supply chain to seamlessly connect with trading partners, improving operational efficiency, increasing transparency and ensuring compliance with growing regulatory requirements.
The accessibility of the Company’s technology platform combined with the comprehensiveness of its product offerings makes TraceLink the best-in-class global track and trace and product serialization vendor on the market today.